|
|
Molecule of the Month October 2007 |
|
|
An Enzyme on the b-Roll: The Crystal Structure of Extracellular Lipase LipA from Serratia marcescens |
||
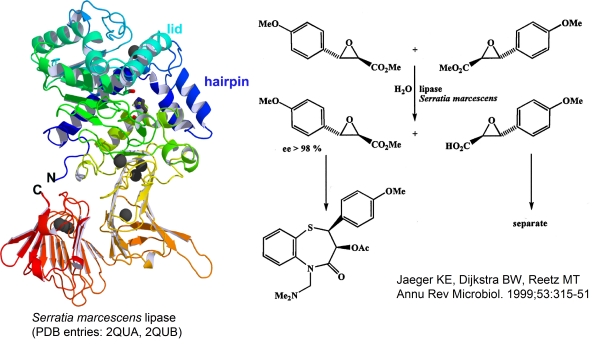
|
||
|
The extracellular lipase LipA is a 65 kDa protein of 613 amino acid residues from gram-negative enteric bacterium Serratia marcescens belonging to lipase family I.3. LipA is a biotechnologically very important enzyme that catalyses with high enantioselectivity the asymmetric hydrolysis of a precursor of diltiazem, a major pharmaceutical for the treatment of heart diseases produced worldwide in an excess of 100 t a-1.
This work was carried out in the group of Prof. Ulrich Baumann. References:
|
||