|
|
Molecule of the Month September 2007 |
|
|
The Structural Basis for Unidirectional Rotation of Thermoalkaliphilic F1-ATPase |
||
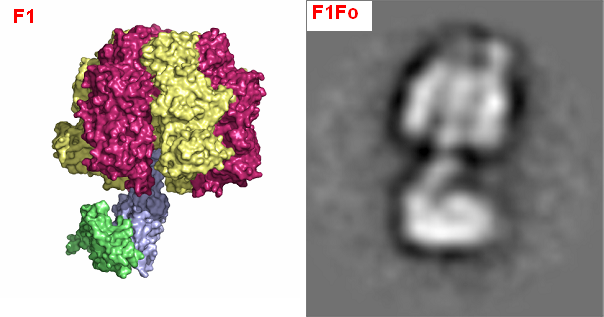
|
||
|
F1Fo-ATP synthase is a twin rotary engine that reversibly couples ion translocation across the membrane via mechanical rotation to the formation of ATP from ADP and inorganic phosphate. The bidirectional operation mode of the F1Fo-ATP synthase requires regulation to control the direction of rotation and its velocity. A unique feature of F1Fo-ATP synthases from alkaliphilic bacilli is a built-in latency of ATP hydrolysis activity. This inhibition of ATPase activity is most pronounced in the ATP synthase from the thermoalkaliphile Bacillus sp. TA2.A1 (TA2F1Fo) and is proposed to be a necessity for the survival of the organism at alkaline pH and high temperature.
This work was carried out in the group of Prof. Achim Stocker. References:
|
||