|
|
Molecule of the Month August 2024 |
|
| Mechanochemistry Facilitates Nitrative Difunctionalization via Radical Ligand Transfer and Electron Catalysis | ||
|
||
|
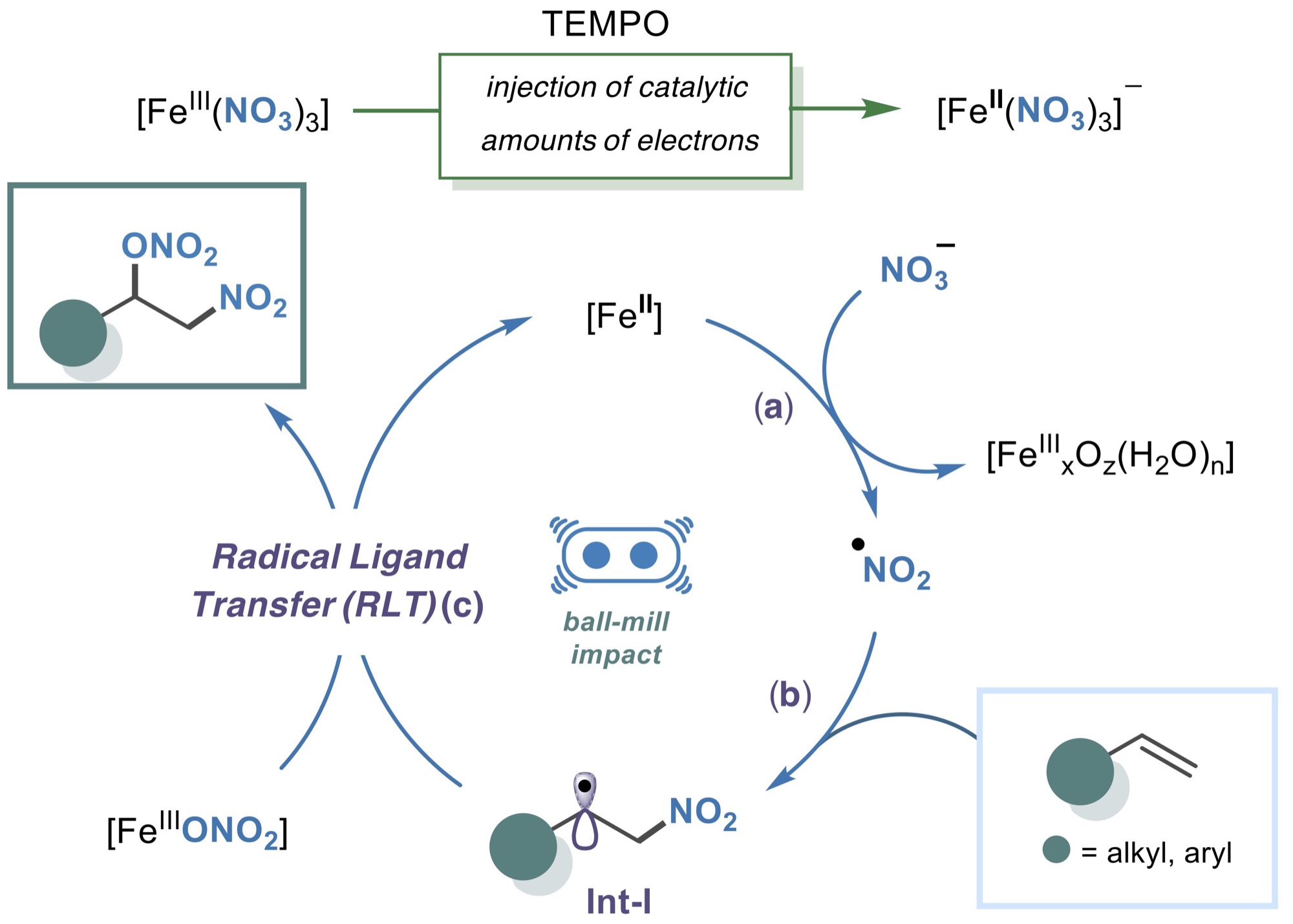
A general protocol is reported for olefin difunctionalization via mechanochemistry, using cooperative radical ligand transfer (RLT) and electron catalysis. Employing mechanochemical force and catalytic TEMPO, ferric nitrate utilizes nitryl radicals to transfer nitrooxy groups via RLT and mediate electron catalysis at room temperature.
This work was carried out in the group of Prof. Dr. Dmitry Katayev. References:
|
||