|
|
Material of the Month June 2019 |
|
| Unravelling Structure - Activity Relationships in Electrocatalysis | ||
|
||
|
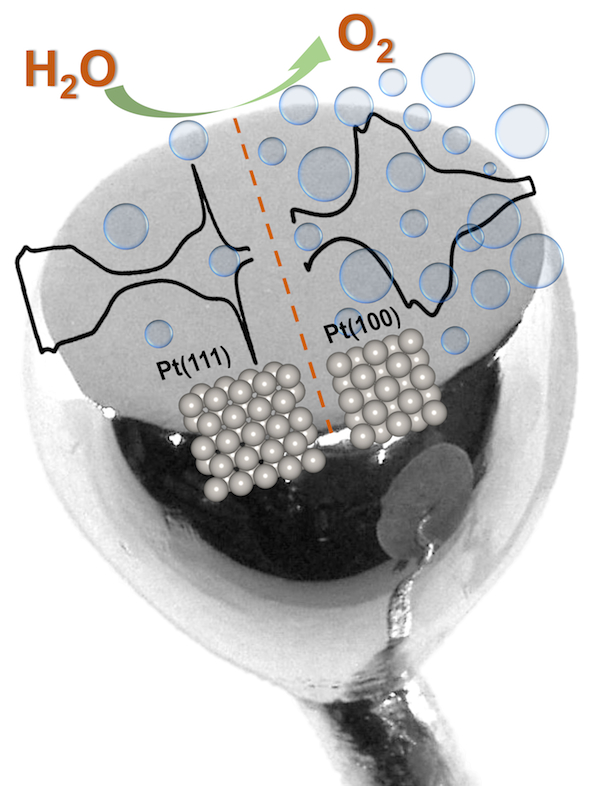
In the electrolysis of water and/or CO2, the anode reaction is the oxygen evolution reaction (OER), which is typically catalyzed by rare, expensive materials. Our recently published work focuses on understanding how the surface structure of the catalyst influences the OER. Combining electrochemistry, in-situ Raman spectroscopy and density functional theory (DFT) calculations we could identify specific surface orientations that exhibit improved OER performance.
This work was carried out in a collaboration between the groups of Prof. Matthias Arenz and Prof. Ulrich Aschauer. References:
|
||