|
|
Molecule of the Month December 2018 |
|
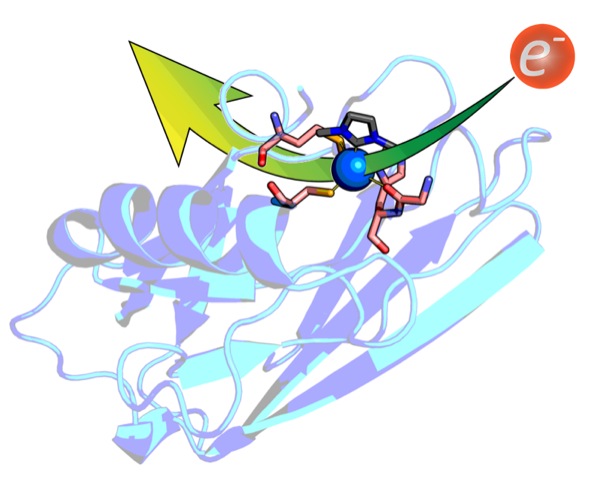
| Re-Thinking the Bonding Mode of Histidine in Metalloenzymes | ||
|
||
|
In metalloenzymes, histidine is typically assumed to bind to metals via a nitrogen atom. We investigated the carbon-bonding mode in the small Cu-protein azurin from P. aeruginosa and, for the first time, demonstrated the implication of such C-bonding by using an N-heterocyclic carbene as a surrogate for C-bound histidine.
This work was carried out in the group of Prof. Dr. Martin Albrecht. References:
|
||