|
|
Molecule of the Month July 2016 |
|
| Structural Insight into Glycopeptide Dendrimer Lectin Interactions | ||
|
||
|
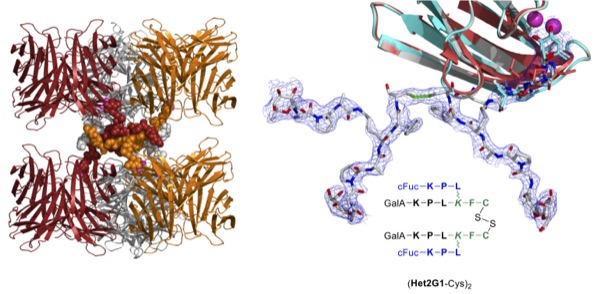
We recently discovered peptide dendrimers with strong activity against multidrug-resistant bacteria such as Pseudomonas aeruginosa and their biofilms. In the course of these investigations we obtained the first high resolution X-ray crystal structure of a peptide dendrimer in form of a complex with the virulence factor LecB, a fucose specific lectin [1]. This structure reveals a supramolecular trimeric structure linked via β-sheets.
This work was carried out in the group of Prof. Jean-Louis Reymond. References:
|
||