|
The adenine analogue 2-aminopurine has been considered as intrinsically fluorescent (τ~11 ns) and is widely used in biochemical assays to probe DNA and RNA structure.[1]
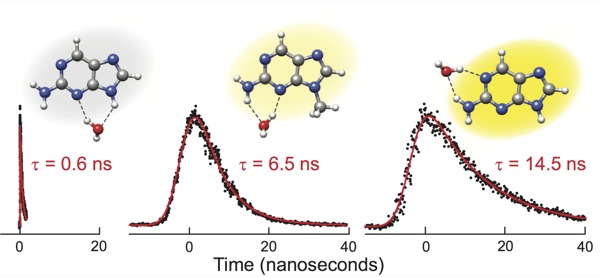
It is now shown that the molecule alone is nearly non-fluorescent with a lifetime of τ=156 ps, however, its fluorescence is increased by up to 95 times through hydrogen bonding to a single water molecule.[2]
This work was carried out in the group of Prof. Samuel Leutwyler.
References:
-
D. C. Ward, E. Reich, L. Stryer;
"Fluorescence studies of nucleotides and polynucleotides"
J. Biol. Chem., 244, 1228-1239, (1969);
http://www.jbc.org/content/244/5/1228.abstract.
-
S. Lobsiger, S. Blaser. R. K. Sinha, H.-M. Frey, S. Leutwyler;
"Switching on the fluorescence of 2-aminopurine by site-selective microhydration"
Nature Chemistry, 6, 989-993, (2014);
doi:10.1038/nchem.2086.
|