|
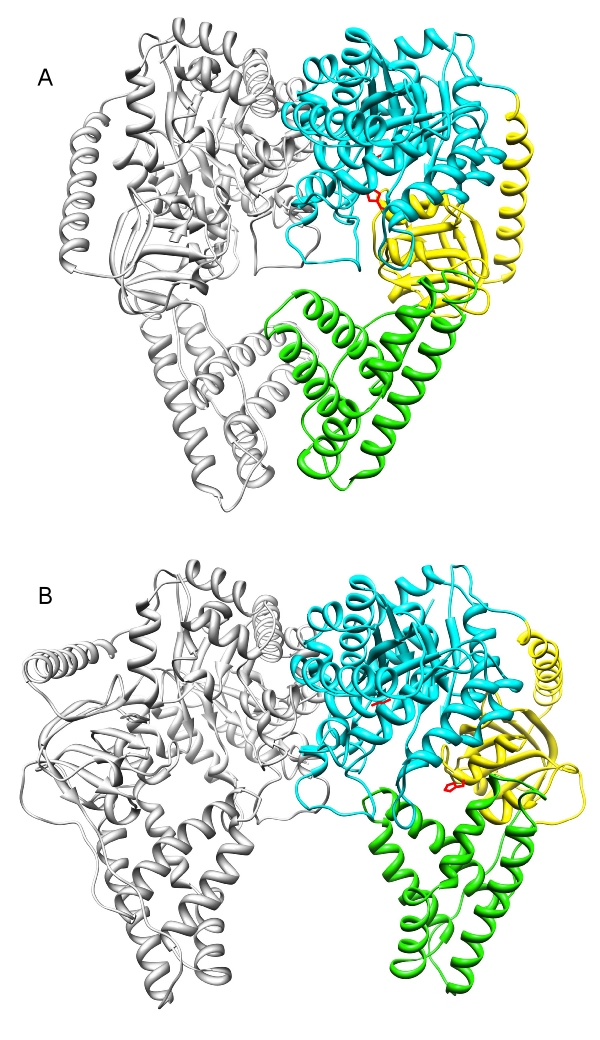
Enzyme I (EI) is the first component of an energy transducing chain that powers the carbohydrate uptake in bacteria. It is one of the most highly conserved proteins without a counterpart in animals. The EI dimer was crystallized in two states:
(A) The swivel domain (yellow) projects into the PEP binding domain (cyan). Phosphate can be transferred from PEP (red) to the active site His (red) of the swivel domain [1].
(B) The swivel domain projects towards the HPr binding domain (green). Phosphate can be transferred to the mobile phosphorylcarrier protein HPr [2].
HPr is not shown in this model because it was not present in the protein crystals, but the EI-HPr complex has been characterized by solution NMR [3].
The movie illustrates the conformation changes of EI during the phosphate transfer from PEP to HPr.
This work was carried out by Philipp Schneider (group of Prof. Bernhard Erni) and Anselm Oberholzer (group of Prof. Ulrich Baumann).
References:
-
A. Teplyakov, K. Lim, P. P. Zhu, G.Kapadia, C. C. Chen, J. Schwartz, A. Howard, P. T. Reddy, A. Peterkofsky and O. Herzberg;
"Structure of phosphorylated enzyme I, the phosphoenolpyruvate:sugar phosphotransferase system sugar translocation signal protein"
Proc. Natl. Acad. Sci. USA, 103, 16218-16223, (2006);
doi:10.1073/pnas.0607587103.
-
A. E. Oberholzer, P. Schneider, C. Siebold, U. Baumann and B. Erni;
"Crystal structure of enzyme I of the phosphoenolpyruvate:sugar phosphotransferase system in the dephosphorylated state"
J. Biol. Chem., 284, 33169-33176, (2009);
doi:10.1074/jbc.M109.057612.
-
J. Y. Suh, M. Cai and G. M. Clore;
"Impact of phosphorylation on structure and thermodynamics of the interaction between the N-terminal domain of enzyme I and the histidine phosphocarrier protein of the bacterial phosphotransferase system"
J. Biol. Chem., 283, 18980-18989, (2008);
doi:10.1074/jbc.M802211200.
|