|
Reactants and reaction products of heterogeneous electron transfer reactions can modify and alter the surface structure and reactivity of electrodes.
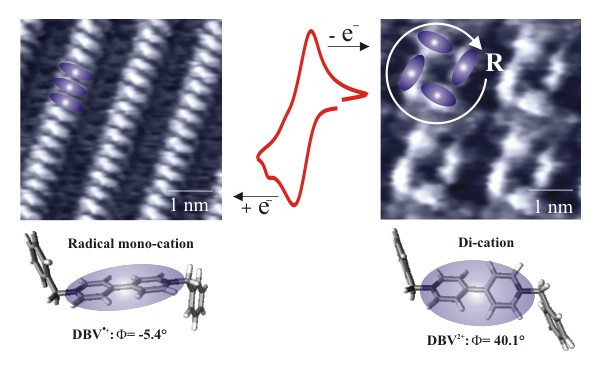
The picture shows distinct structural transitions within 2D crystalline viologen phases adsorbed on a chloride-modified Cu(100) electrode surface. Di-cationic dibenzylviologens (DBV2+) order themselves on the Cu(100)-c(2 x 2)-Cl lattice into chiral assemblies of 4 DBV2+ads molecules enclosing a 1 nm cavity in their center. More compact (DBV·+ads)n stacking phases are observed in the in-situ STM experiment for the mono-reduced dibenzylviologens (DBV·+). Both surface structures can reversibly be transformed into each other by the electron transfer reaction.
This work was carried out in the Interfacial Electrochemistry Group of Dr. Peter Broekmann.
References:
-
D.-T. Pham, S.-L. Tsay, K. Gentz, C. Zoerlein, S. Kossmann, J.-S. Tsay, B. Kirchner, K. Wandelt and P. Broekmann;
"Quasi-Reversible Chloride Adsorption/Desorption through a Polycationic Organic Film on Cu(100)"
J. Phys. Chem. C, 111, 16428-16436, (2007);
doi:10.1021/jp073469q.
-
S. Breuer, T. D. Pham, S. Huemann, R. Hunger, K. Wandelt and P. Broekmann;
"Organic layers at metal/electrolyte interfaces: molecular structure and reactivity of viologen monolayers"
N. J. Phys., 10, 125033/1-24, (2008);
doi:10.1088/1367-2630/10/12/125033.
|