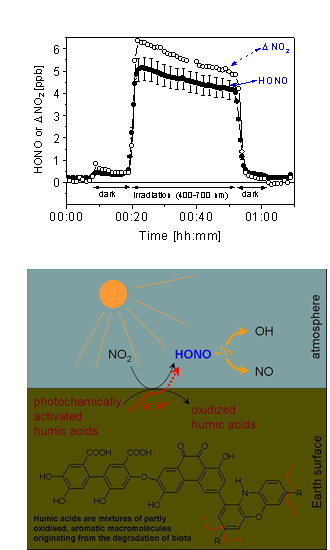
|
Nitrous acid (HONO) is a photochemical precursor of the hydroxyl radical which is the key oxidant in the degradation of most air pollutants in the troposphere.
In laboratory experiments we exposed surfaces containing traces of humic acids or soil surfaces to visible or UV light to simulate solar irradiation. We observed an efficient transformation of gaseous NO2 into HONO on these light activated surfaces. Our figure shows the results from an experiment, where we irradiated a layer of humic acid (8 µg cm-2) in the 400-700 nm wavelength range while a flow of synthetic air containing 20 ppb NO2 passed through the reactor. During the irradiation we observed a substantial loss of NO2 and a nearly as strong formation of HONO, which is enhanced by a factor of 30 as compared to production in the dark.
Since such surfaces represent a strong absorber of terrestrial solar light and surfaces containing traces of humic substances are abundant in the environment, the presented reaction could explain the high daytime concentrations of nitrous acid, observed in the boundary layer, which can account for up to 60% of the integrated hydroxyl radical source strengths in the lowest atmosphere.
This work was carried out in the group of Prof. Heinz W. Gäggeler.
References:
-
K. Stemmler, M. Ammann, C. Donders, J. Kleffmann, C. George;
"Photosensitized reduction of nitrogen dioxide on humic acid as a source of nitrous acid"
Nature, 440, 195-198, (2006);
doi:10.1038/nature04603.
|