|
|
Molecule of the Month August 2005 |
|
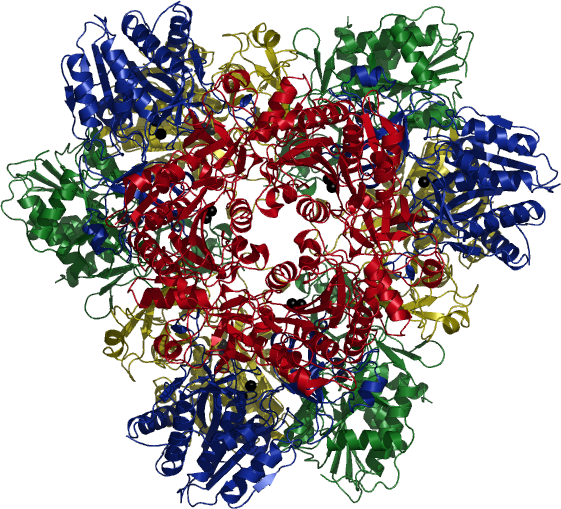
| Tetrahedral Aminopeptidase from P. horikoshii | ||
|
||
|
The protein content of every living cell is constantly renewed through synthesis of new proteins and degradation of unneeded or misfolded proteins. The only very recently discovered tetrahedral aminopeptidases seem to fulfil a key role in the degradation pathway of bacteria and archaebacteria.
This work was carried out by Santina Russo in the group of Prof. Ulrich Baumann. References:
|
||