|
|
Molecule of the Month April 2015 |
|
| Interactions of Arene Ruthenium Metallaprisms with Human Proteins | ||
|
||
|
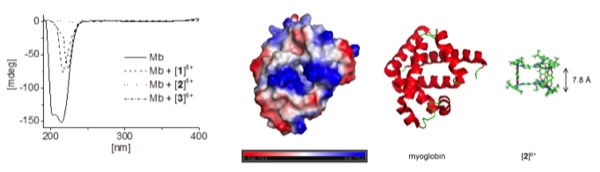
Interactions between hexacationic arene ruthenium metallaprisms and the human proteins human serum albumin, transferrin, cytochrome c, myoglobin and ubiquitin have been studied using NMR spectroscopy, mass spectrometry and circular dichroism spectroscopy.
This work was carried out in the group of PD Dr. Julien Furrer. References:
|
||